
題目列表(包括答案和解析)
①H2(g)+![]() O2(g)
O2(g)![]() H2O(g) ΔH=-241.8 kJ·mol-1
H2O(g) ΔH=-241.8 kJ·mol-1
②2H2(g)+O2(g)![]() 2H2O(g) ΔH=-483.6 kJ·mol-1
2H2O(g) ΔH=-483.6 kJ·mol-1
③H2(g)+ ![]() O2(g)
O2(g)![]() H2O(l) ΔH=-285.8 kJ·mol-1
H2O(l) ΔH=-285.8 kJ·mol-1
④2H2(g)+O2(g)![]() 2H2O(l) ΔH=-571.6 kJ·mol-1
2H2O(l) ΔH=-571.6 kJ·mol-1
則氫氣的燃燒熱為( )
A.241.8 kJ·mol-1 B.483.6 kJ·mol-1 C.285.8 kJ·mol-1 D.571.6 kJ·mol-1
已知熱化學方程式:
① C2H2(g) + O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) == CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ 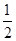 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
則反應④ 2C(s)+ H2(g) == C2H2(g)的△H為( )
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
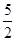 O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) == 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1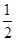 O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1
O2(g) == H2O(1) △H3 =" -285.8" kJ·mol-1| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
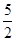 O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
已知熱化學方程式:
① C2H2(g) + O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
O2(g) ="=" 2CO2(g)+H2O(l) ΔH1=-1301.0 kJ?mol-1
② C(s)+ O2(g) ="=" CO2(g) △H2=-393.5 kJ?mol-1
③ H2(g)+ O2(g) ="=" H2O(1) △H3 =" -285.8" kJ·mol-1
則反應④ 2C(s)+ H2(g) ="=" C2H2(g)的△H為
| A.+228.2 kJ·mol-1 | B.-228.2 kJ·mol-1 |
| C.+1301.0 kJ·mol-1 | D.+621.7 kJ·mol-1 |
湖北省互聯(lián)網(wǎng)違法和不良信息舉報平臺 | 網(wǎng)上有害信息舉報專區(qū) | 電信詐騙舉報專區(qū) | 涉歷史虛無主義有害信息舉報專區(qū) | 涉企侵權舉報專區(qū)
違法和不良信息舉報電話:027-86699610 舉報郵箱:58377363@163.com